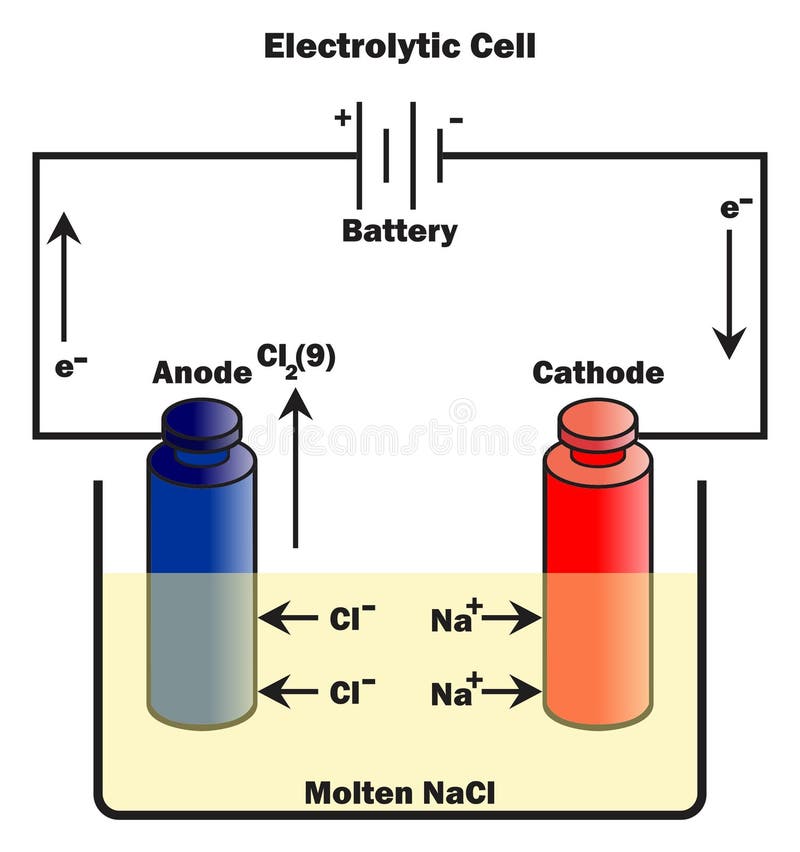
Neat Info About Why Do Electrons Always Flow From Anode To Cathode
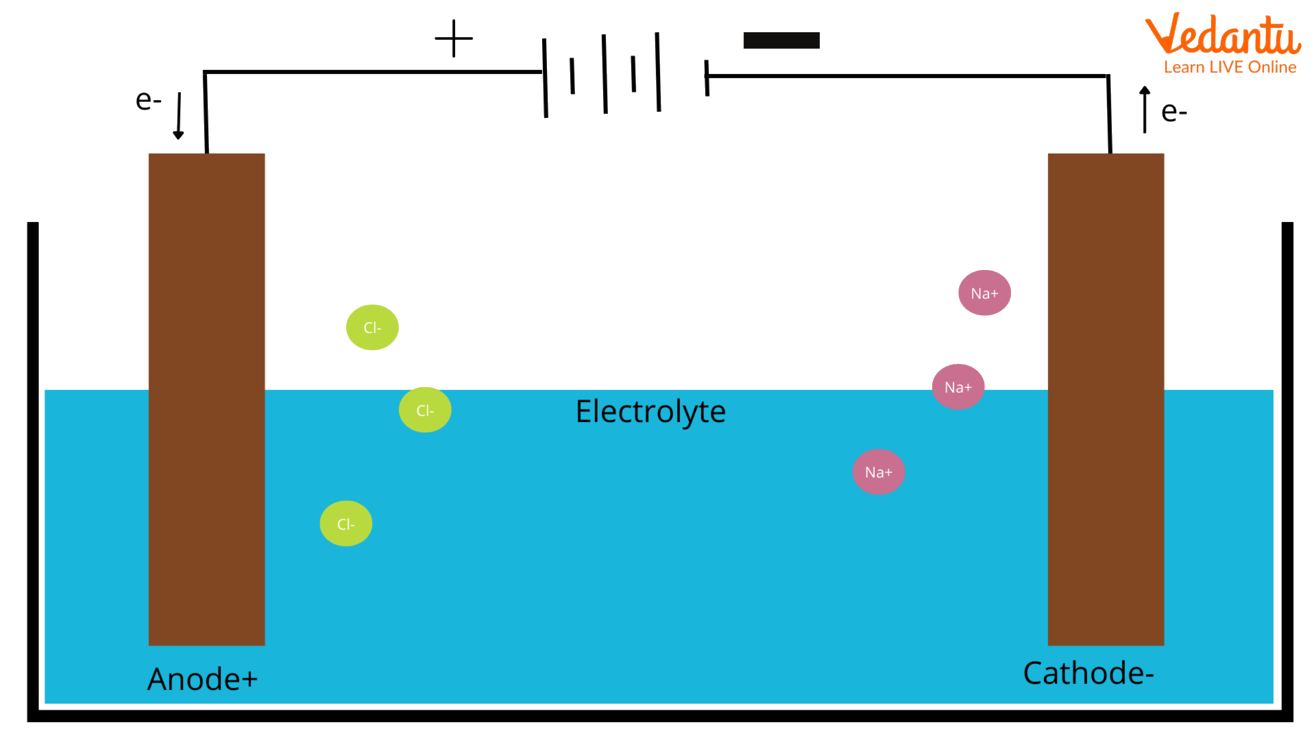
Electrochemistry Which Is Anode And Cathode?, 57 OFF
Electrons on the Move
1. The Curious Case of Anode to Cathode
Ever wonder why electrons, those tiny negatively charged particles that power pretty much everything, seem to have a preferred direction in circuits? It's not like they're following some cosmic GPS, but there's a fundamental reason why they generally flow from the anode to the cathode. Think of it like this: Imagine you're at the top of a water slide. Naturally, you're going to slide down, right? Electrons, in a way, are doing the same thing. They're just "sliding" down an electrical potential.
The key here lies in understanding what the anode and cathode actually are. The anode is the electrode where oxidation occurs. Oxidation, in simple terms, is where a substance loses electrons. The cathode, on the other hand, is where reduction happens; that's where a substance gains electrons. So, if electrons are being lost at the anode and gained at the cathode, it's a pretty logical journey for them to take, isn't it? They're simply moving from a place where there's a surplus to a place where there's a deficit.
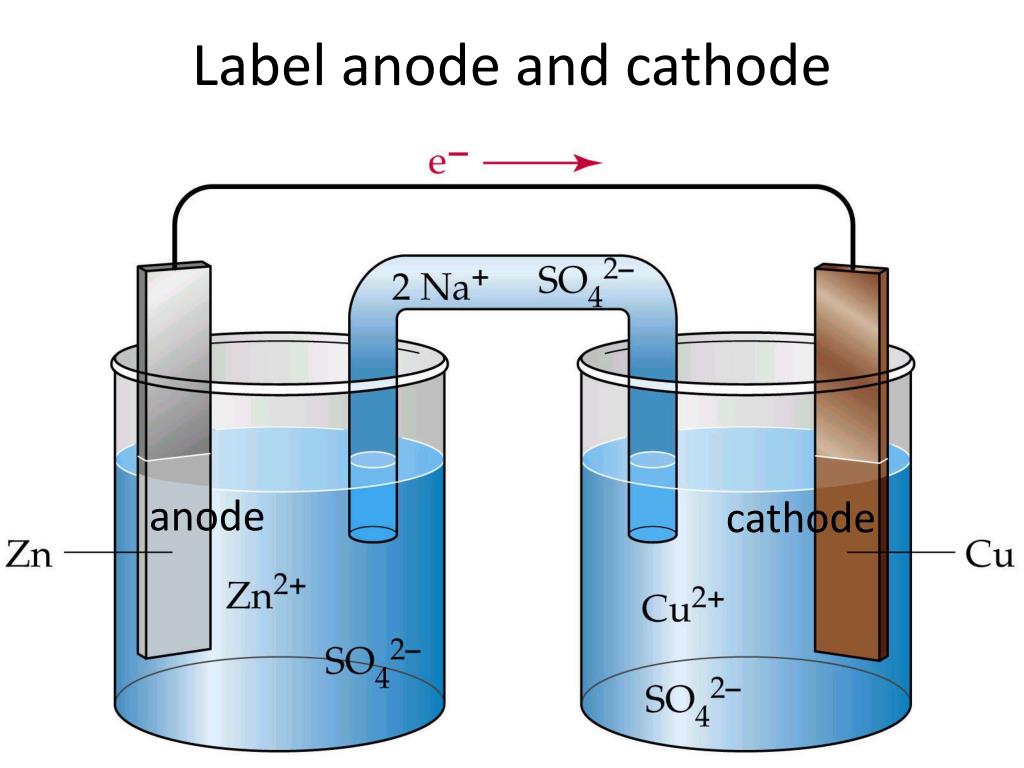
Consider a simple battery. Inside, chemical reactions are happening constantly. At the anode (often the negative terminal), a material is being oxidized, releasing electrons. These electrons then travel through the external circuit — the wire, the lightbulb, whatever's connected to the battery — towards the cathode (usually the positive terminal). At the cathode, another chemical reaction is occurring, consuming those electrons. And thus, the circuit is complete and your device works. Yay, science!
Now, some might quibble about "conventional current," which historically was defined as flowing from positive to negative. That's a whole other story, stemming from before we fully understood what electrons were. But when we're talking about the actual movement of electrons, it's consistently from the electron-rich anode to the electron-deficient cathode. It's a one-way electron highway!
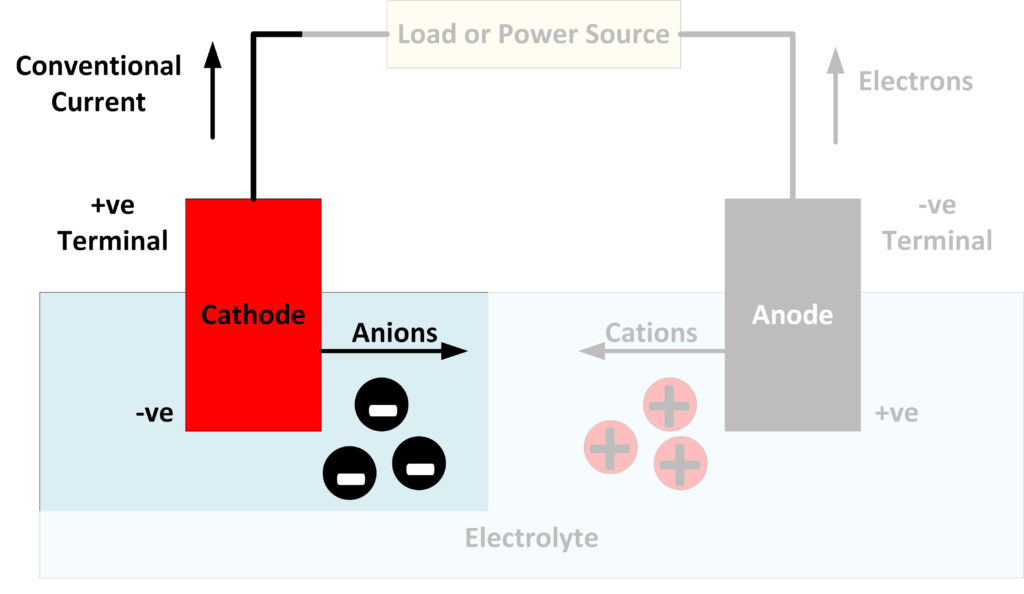
What Is A Cathode? Battery Power Tips
Anode vs. Cathode
2. Defining the Electrical Players
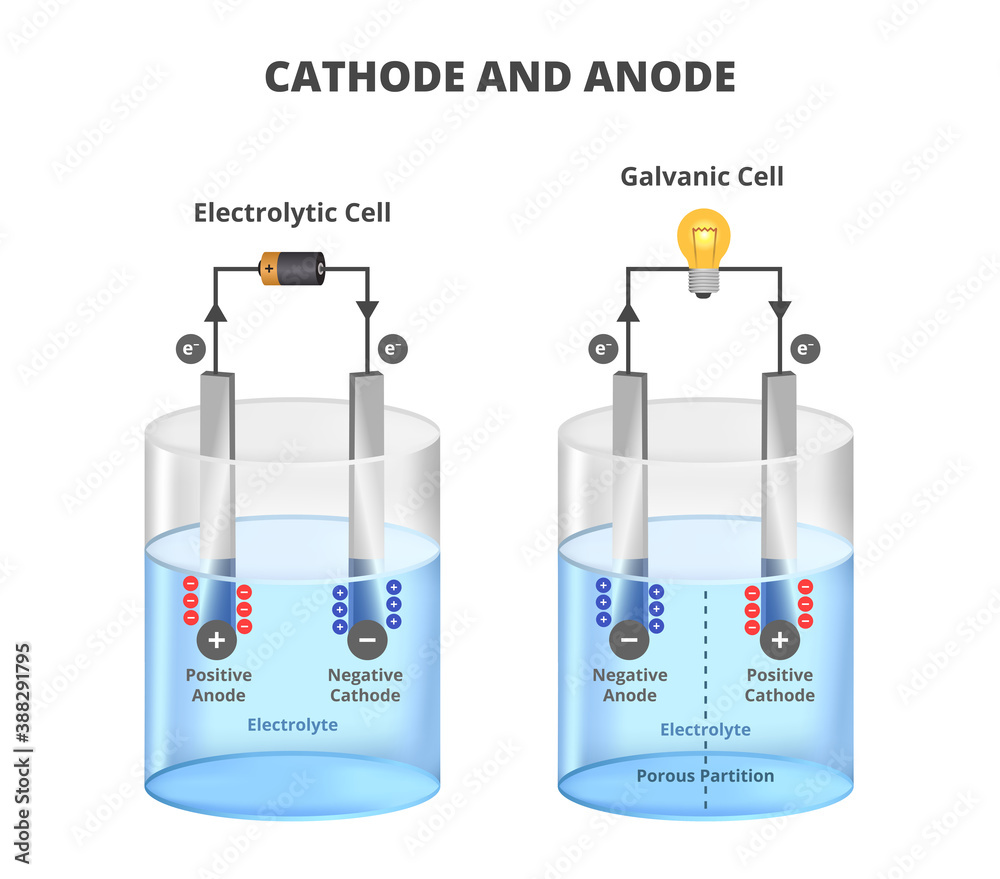
To really get to the heart of this electron flow, let's clearly define our terms. The anode, as mentioned before, is the electrode where oxidation happens. Think of it as the electron giver. It's losing electrons to the circuit. In a battery during discharge, the anode is usually the negative terminal. In an electrolytic cell, it's the positive terminal — confusing, I know, but it depends on whether the device is supplying or consuming energy.
Conversely, the cathode is the electrode where reduction takes place. This is the electron taker. It's accepting electrons from the circuit. In a discharging battery, the cathode is the positive terminal. And, just like the anode, in an electrolytic cell, it flips and becomes the negative terminal. Essentially, the cathode provides a place where electrons can be welcomed and utilized, completing the circuit's journey.
The difference in electrical potential between the anode and the cathode is what drives the electron flow. This potential difference creates an electric field, which exerts a force on the electrons, causing them to move. The higher the potential difference, the stronger the electric field, and the faster the electrons will flow. Its like the steeper the water slide, the faster you'll go down!
It's important to remember that the terms "anode" and "cathode" are defined by the reaction happening at the electrode, not by the polarity. The polarity (positive or negative) can change depending on whether the device is providing power (like a battery) or consuming power (like an electrolytic cell). So, focus on the electron movement: electrons leave the anode and arrive at the cathode, no matter the sign.
The Role of Electrical Potential
3. The Driving Force Behind Electron Migration
Why do electrons want to move from the anode to the cathode? The answer lies in electrical potential, also known as voltage. Electrons, being negatively charged, are attracted to areas of positive charge and repelled by areas of negative charge. This attraction and repulsion create an electric field, which exerts a force on the electrons. The anode has a lower electrical potential than the cathode. This "potential difference" is what drives the electrons through the circuit.
Think of it like a hill. Objects naturally roll downhill from a point of higher potential energy to a point of lower potential energy. Similarly, electrons "roll" from a point of lower electrical potential (the anode) to a point of higher electrical potential (the cathode). This movement minimizes the system's overall energy, making it a more stable state.
This potential difference isn't just some abstract concept. It's what allows us to power our devices. A battery, for example, maintains a relatively constant potential difference between its terminals through chemical reactions. This ensures a continuous flow of electrons as long as the battery is connected to a circuit and the reactions can continue.
Without a difference in potential, electrons would just sit there, not doing much of anything. It's the electrical potential that gives them the "push" they need to move through the circuit, delivering the energy that powers our world. So, electrical potential is the silent hero behind every electronic device.
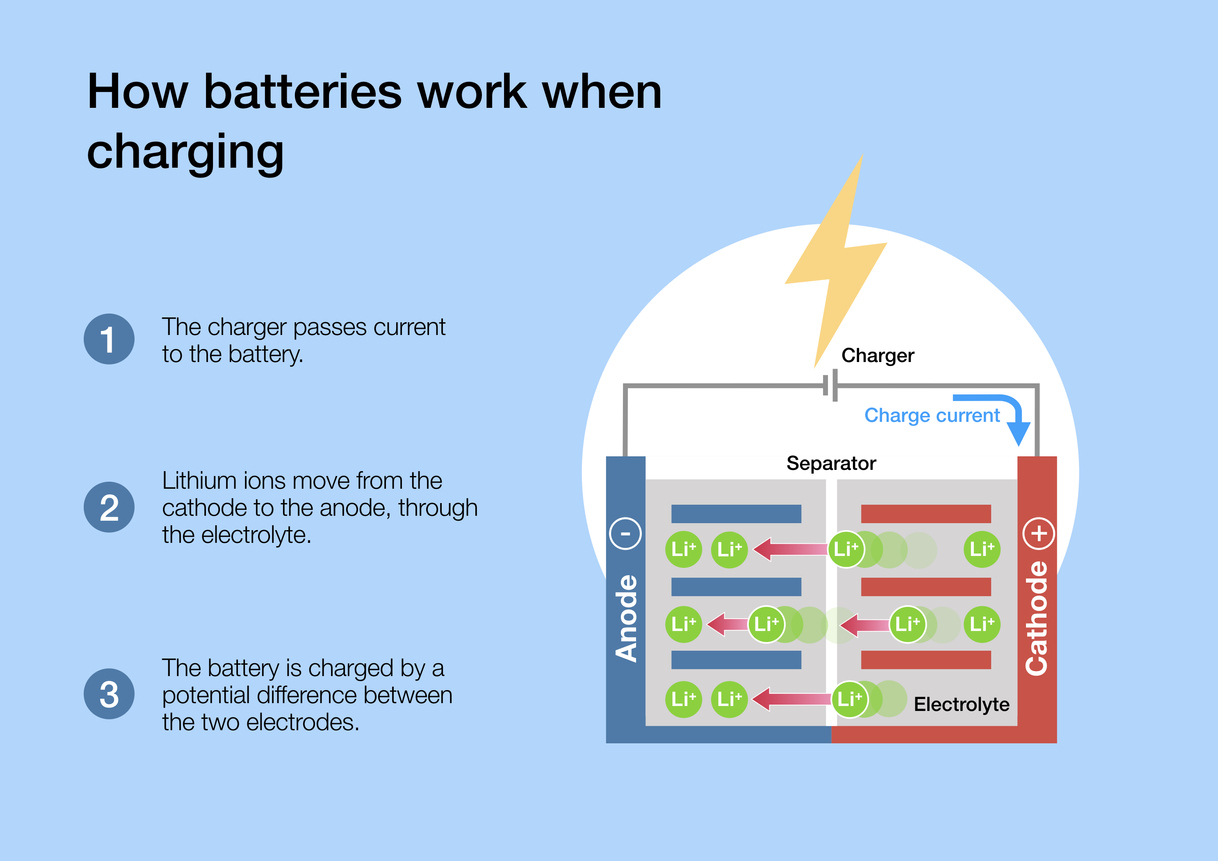
Beyond Batteries
4. Electron Movement in Various Contexts
While batteries are a great example, the anode-to-cathode electron flow principle extends far beyond just powering your flashlight. Consider a simple LED (light-emitting diode). When you apply a voltage to the LED, electrons flow from the cathode (typically the shorter lead) to the anode (typically the longer lead). This flow of electrons through the semiconductor material causes it to emit light. No electron flow, no light! It's that simple.
Electroplating is another fantastic example. In this process, we want to coat a metal object with a thin layer of another metal. The object to be coated is made the cathode, while the metal we want to coat with is the anode, both submerged in an electrolyte solution. As electrons flow from the anode to the cathode through the solution, metal ions from the anode are reduced at the cathode, depositing a thin layer of the desired metal onto the object. Its like a tiny, controlled metal rain!
Even in vacuum tubes (remember those?), electrons flow from the cathode (often a heated filament that emits electrons) to the anode (a positively charged plate). This flow can be controlled by other elements in the tube to amplify signals or perform other electronic functions. These principles still find use in niche applications and are fundamental to understanding the history of electronics.
The common thread in all these examples is the consistent movement of electrons from the anode, where they are released, to the cathode, where they are accepted. Whether it's lighting up a bulb, coating a piece of metal, or amplifying a signal, the underlying principle remains the same: electrons making their purposeful journey from anode to cathode.
Common Misconceptions & FAQs about Electron Flow
5. Clearing Up Electron-Related Confusion
Sometimes, understanding electron flow can feel a bit like navigating a maze. One common misconception is that electrons travel incredibly fast through wires. While they do move very quickly on an atomic level, the actual "drift velocity" of electrons in a circuit is surprisingly slow — often just a few millimeters per second. The electrical signal itself travels much faster, almost at the speed of light.
Another point of confusion is the "conventional current" direction, which, as mentioned earlier, is the opposite of the actual electron flow. This is a historical artifact, and while it can be confusing, it's important to remember that the fundamental principle of electron movement from anode to cathode remains valid regardless of which "current" you're talking about. Just remember, electrons are the real MVPs, and they flow from negative to positive (anode to cathode).
Furthermore, people often think that the anode is always the negative terminal. This is true for a discharging battery, but not necessarily true for other devices like electrolytic cells. Always remember the definition of anode and cathode based on the chemical reaction (oxidation and reduction), rather than simply relying on the sign of the terminal.
6. Frequently Asked Questions
Q: Why don't electrons flow from cathode to anode?
A: Because the cathode is already accepting electrons! It's the site of reduction. The anode is the one that releases electrons through oxidation. The electrical potential drives them from the anode (lower potential) to the cathode (higher potential).
Q: Is electron flow the same as current?
A: Yes and no. Electron flow is current, but "conventional current" is defined as flowing in the opposite direction (positive to negative). When discussing the actual movement of electrons, yes, electron flow is from anode to cathode and constitutes an electric current.
Q: Does the type of material in the anode and cathode affect electron flow?
A: Absolutely! The material determines the ease with which oxidation and reduction can occur. Some materials readily release or accept electrons, resulting in a greater potential difference and a stronger electron flow. This is why specific materials are chosen for batteries and other electrochemical devices.